Living a Fluorine Future
NASA, lithium batteries, the United States Army and surgical gloves are more related than you may think. They each tie back to a white, solid substance named CFX, synthesized at Rice University at over 600 degrees Celsius in a nearly explosive reaction. This precarious-sounding compound is much less harmful than it sounds. In fact, since its discovery in 1965, it has proven to be surprisingly versatile and valuable.

CFX was discovered by Rice chemist John Margrave, a man who revolutionized chemistry and produced what his Ph.D. advisor, Robert Gilles, once called, “an astonishingly outstanding quality and quantity of scientific work.” CFX, a highly durable lubricant and coating, is now found all over the world, from spacecraft to hospitals to batteries. It stands as just one of Margrave’s many breakthroughs in the realm of fluorine chemistry.
Margrave’s interest in fluorine chemistry spanned decades and scientific disciplines. From pioneering calorimetry methods and reactive gas-phase species to breakthrough coatings like CFX, his career unfolded as a sustained exploration of chemistry under extreme conditions.
Margrave was initially drawn to fluorine chemistry for its obscurity. As he told the Rice University Review in 1971, “Fluorine was probably among the least understood of the so-called halogen family. I was interested in the fact that it was a poorly known element, that it was difficult to work with, and that it was very reactive.” Upon his arrival at Rice in 1963, he dove straight into fluorine’s mystery, focusing on the discovery and classification of new fluorine compounds.
At the time, chemists across the world were captivated by the idea of using fluorine as an alternative to oxygen in producing rocket fuel. Since fluorine reacts even more violently than oxygen, it seemed worth investigating. Margrave and his graduate student Richard Lagow ’70 began testing fluorine’s reactions with a wide range of elements and compounds in search of a promising fuel.
In one of these experiments, reacting fluorine with carbon produced a white, solid substance reminiscent of one developed in Germany in the 1930s. At first, Margrave and Lagow thought they had found something unoriginal, but they soon realized they had something new. Their compound was significantly more stable. “We could take a small pile of the stuff and hit it hard with a hammer and nothing would happen,” Margrave told the Rice Review. “After all sorts of extremely tough tests, it did not explode.”

They named the compound CFX, with “C” for carbon, “F” for fluorine, and “X” representing the ratio of fluorine to carbon in the compound. Not only did CFX not break or explode under force and pressure, but it also did not burn in air, did not melt (even at 600 degrees Celsius), and resisted corrosion and gas permeability. It was a significant improvement over Teflon, the previous gold standard, which softened at 350 degrees Celsius and even burned at 400 degrees Celsius. This made CFX a valuable high-temperature lubricant, perfect for mechanical components in extreme conditions, such as moving parts in rockets, planes and ships, particularly near the engine.
Margrave quickly informed the US Air Force, the US Army and NASA of CFX’s superior lubrication qualities. All three immediately saw its promise. For a brief while, Margrave worked with these organizations to test CFX, seeking to maximize performance under extreme conditions. CFX soon began replacing less effective graphite lubricants in military and aerospace applications.
CFX was more than just a phenomenal lubricant. It also made a phenomenal coating for many organic materials, like rubber. Margrave and Lagow found that coating plastic or rubber items with a 0.1 mm thick layer of CFX created something “impervious to most solvents” and resistant to most extreme conditions. Margrave proposed its use for surgical gloves to increase protection for personnel working with animals or tissue that had been exposed to toxic substances. The idea took off, and Margrave and his team filed patents for fluorocarbon-coated surgical gloves, boots and protective plastic clothing. They called their method the LaMar Process — a blend of their last names — and named the coating FluoroKote.
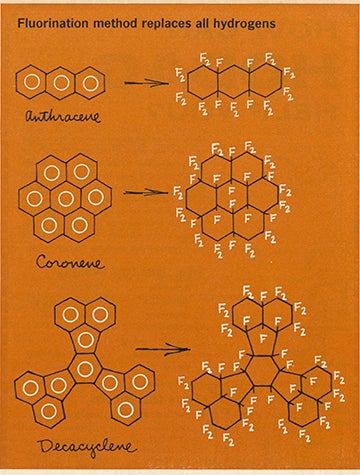
By the 1980s, CFX had drawn international attention. Japan Chemistry Week praised its versatility: “Because of their unique properties, fluorine chemicals have been expanding and now cover drug and farm chemical intermediates, apparel and household-use products, automobiles, construction and the electrical parts, space, aircraft, and atomic power industries.”
Part of what made CFX so impactful was the comparative ease of its production. Other chemists needed long, multi-step reactions to synthesize fluorinated hydrocarbons. In contrast, Margrave’s direct, one-step fluorination approach was quick, efficient and remarkably safe. It had an almost 100 percent success rate, allowing it to be produced in large quantities and making CFX affordable. FluoroKote was sold for $10 per gram and quickly found its way into global industrial, medical and consumer markets.
The discovery of CFX ultimately became the crown jewel of Margrave’s long and productive career — but it was far from his only accomplishment in fluorine chemistry. Long before CFX, he had already begun making key contributions to the field, including the study of exotic compounds like carbene analogs.
Throughout the 1950s and 1960s, Margrave investigated highly reactive intermediates known as carbenes, which are difficult to study because of their short lifetimes. The basic carbene (CH2) lasts only a few microseconds — too brief for any scientist to get a good look at it. But Margrave found that fluorinated versions like CF2 lasted longer and that silicon difluoride (SiF2) was surprisingly stable: its monomeric form had a half-life of about 150 seconds.
These two-and-a-half minutes were a chemistry treasure: they provided time for Margrave and his team to determine SiF2’s physical properties, measuring its bond angles, bond lengths and dipole moment. “His splendid intuition has enabled him to synthesize ideas from several branches of chemistry and to initiate new areas of science,” Gilles wrote. These studies extended to SiF2’s reactions with electron donors, electron acceptors and a wide variety of inorganic and organic molecules — work that became foundational in the study of reaction mechanisms and helped expand the possibilities of fluorine chemistry.
In parallel with this work, Margrave pioneered techniques like fluorine bomb calorimetry and mass spectrometric characterization of high-temperature gaseous species, particularly for metallic fluorides. He also developed multisided matrix isolation infrared spectroscopy to study transient fluorinated species in the gas phase — a breakthrough that allowed for high-precision, real-time monitoring of unstable compounds.

In his later years at Rice, Margrave turned his attention to the newly discovered fullerenes, including C60, commonly known as the buckyball. He quickly recognized the potential of fluorinating these nanostructures. Collaborating with colleagues including Richard Smalley, Robert Hauge and Edward Billups, Margrave explored how fluorine could be used to functionalize fullerenes and carbon nanotubes.
Fluorine, often shunned by chemists for its extreme reactivity, proved to be a powerful tool for modifying carbon nanostructures. Margrave and his collaborators developed techniques to fluorinate fullerenes and carbon nanotubes, enabling a wide range of follow-up chemical reactions and yielding dozens of soluble, functional derivatives, including hydrotubes, methoxy nanotubes and nylon-like polymers.
One of the team’s most notable achievements was discovering that fluorinated nanotubes could be thermally cleaved into ultra-short fragments — some as small as 50 nanometers — which were much more soluble and suitable for biomedical, electronic and energy applications. “Compared to other methods of forming derivatives of carbon nanostructures, fluorination leads to reactions that are more general in nature and more easily extrapolated to a macro or production scale,” Margrave told Rice News in 2002.
Billups recalled that Margrave never argued with fellow researchers and maintained the utmost calm and politeness even in the face of frustration. “I don't have anything negative to say about Margrave,” Billups said. “And, you know, that’s a pretty substantial statement.” He added: “He was quiet. He was talented. He was excellent in every way, easy to communicate with. I never heard him raise his voice. That tells you something.”
Margrave’s work spanned from the fleeting existence of reactive intermediates to stable, world-changing materials like CFX and the cutting edge of nanotechnology. But his legacy goes beyond his discoveries.
He was widely admired for his mentorship and generosity. “John Margrave is a superstar scientist with great ideas, who appreciates creativity and gives his students and postdoctoral research associates great personal scientific freedom to excel in many areas,” one of his students recalled. “He creates a wonderful and relaxed research environment with exotic equipment and novel techniques. Margrave’s door is always open; knock and you will receive wise counsel, worthwhile encouragement and practical suggestions.”
Among his colleagues, Margrave was known for his warmth, clarity of thought and ability to jump straight to the heart of a complex problem without getting bogged down in details. As Gilles wrote, “I suspect that he is rigorous in his chemistry, forthright and innovative in his approaches, stimulating in his lectures and discussions, and gentle in his interactions.”
He also believed deeply in public engagement with science. His list of “Ten Reasons Why I Love Chemistry!” sought to make chemistry accessible and intriguing for children. His chemistry magic show, lovingly titled “Meaningful Manipulations of Millions of Madly Moving Molecules,” began as a party trick for his then-six-year-old son and grew into a popular outreach program sponsored by the American Chemical Society.

Though fluorine chemistry remained central to his work, Margrave’s research also extended into metal carbenes, laser-material interactions and vapor deposition techniques, reflecting a drive to understand matter at its most reactive and extreme.
Margrave received many honors over his career, including election to the National Academy of Sciences, three R&D100 awards, and the ACS Award in Fluorine Chemistry. Yet he remained humble.
Margrave’s own words serve as an appropriate summary of his character: “One should expect the best from people, avoid personal conflicts, be friendly, be ambitious, be open-minded, be venturesome, be meticulous, be skeptical. One should not get mad and should not write poems. Be versatile and recognize new opportunities.” And, quoting Mark Twain, he continued, “Even if you’re on the right track, you’ll get run over if you just sit there!”
Above all, Margrave’s career tells a story of how the small-scale, elusive, mesmerizing experiments of a Rice University lab can make their way across the world and into slivers of everybody’s daily life. We often separate scientific research from our lived experience without considering how it shapes our world. Perhaps you have put on a pair of surgical gloves during a high school chemistry lab. Perhaps you have visited NASA’s Rocket Park to gawk at engines twice your height or powered up a device using a lithium battery. For each of these moments, and countless more, Margrave and CFX may have touched your life without you even knowing it.
The 1970 Rice Review said it best: “There will undoubtedly be fluorine in your future, and it may be there because of research done at Rice University.” Thanks to Margrave’s drive and curiosity, we are each living that future now.
— Sasha Miller ’28
This work was made possible with support from the Fondren Fellows program.
