Where the Elements Begin
“We’re not only stardust, we’re fallout,” Stanford Woosley ’66 ’71 said, reflecting on the legacy of his former advisor, Donald Clayton. It is a statement that captures the essence of Clayton’s pioneering work: that the iron in our blood and the nickel embedded in meteorites were not only forged in stars, but released into space through violent supernovae as radioactive isotopes.

Clayton spent his career pursuing this idea, developing the theoretical frameworks to explain it, predicting the signals by which it could be confirmed and mentoring the students who would help prove it. When the first detection of gamma-ray lines from supernovae was made in 1987, it validated Clayton’s long-held vision and launched a new branch of observational astronomy.
Clayton began graduate school at Caltech in 1957 just as his advisor — and future Nobel laureate — William Fowler was co-writing the now-famous B2FH paper on the synthesis of elements in stars. Clayton read an early “sneak preview” of the full paper and was captivated.
“The thought that the chemical elements of our world may have had a natural origin seized me,” he later wrote in his autobiography. “In my science education concerning the chemical elements, the question of why they existed had never arisen. I had grown up in a deeply religious family that took it for granted that God had created everything just as it is.”

Hoping to join Fowler’s research group, Clayton delayed beginning his own research until Fowler returned from sabbatical in his second year. “Those fortunate choices launched my career,” he noted.
Building on the questions that first captured his attention in graduate school, Clayton would go on to focus his career on exploring the creation of the chemical elements within stars, a field known as nucleosynthesis, with particular emphasis on the stellar processes that synthesize new atomic nuclei.
In their cores, stars fuse hydrogen into helium and, over time, produce progressively heavier elements such as carbon, oxygen, and silicon, up to iron. Elements heavier than iron, including gold, platinum and lead, cannot be formed through fusion in stellar cores. Instead, they are produced through other processes, such as neutron capture, that occur in late stellar evolution, in stellar explosions called supernovae or in merging neutron stars.
“The idea in those days that everything heavier than helium, essentially, was made in stars was still novel,” said Woosley, who is now a professor emeritus of astronomy and astrophysics at the University of California at Santa Cruz. Understanding how and when these elements form was one of the central questions of 20th-century astrophysics.

In 1963, Clayton joined the newly established Department of Space Science at Rice where he would continue to explore nucleosynthesis in his own research group. The department itself was a response to national ambition. Following President John F. Kennedy’s speech at Rice Stadium declaring the United States’ intent to reach the Moon, Rice President Kenneth Pitzer sought to demonstrate the university’s commitment to space exploration. When Physics and Geology declined to lead the effort, Pitzer created a new department with remarkable speed. The founding faculty included chairman Alexander Dessler, Brian O’Brien, Curt Michel and Clayton. Bob Haymes, whose early work focused on atmospheric neutrons, joined the following year to pursue gamma-ray astronomy. It was an environment built for ambition — a place where big ideas could be tested and new fields could take root.
One of those ideas emerged from a conversation between Clayton and Haymes. Haymes posed a question: Does stellar nucleosynthesis produce sources of gamma rays by producing radioactivity? Clayton described a theoretical model in which supernova explosions generate some of the heaviest elements in the universe by bombarding atomic nuclei with large numbers of neutrons. These newly formed, unstable isotopes must be explosively ejected into space for the process to work properly. Earlier researchers had even speculated that the radioactive decay of such isotopes might be the cause of the slow, 60-day decline in visible light observed after a supernova, first documented by Chinese astronomers in 1054. Some had proposed that a radioactive isotope of californium might be responsible.
That night, Clayton began calculating the gamma-ray flux such a supernova might produce. He showed that if enough radioactive material were present to explain the gradual fading of visible light over 60 days, the same decay would emit gamma rays potentially strong enough to be observed from Earth.
His graduate student, Wade Craddock ’67, calculated the gamma-ray flux for a variety of isotopes. The strongest signal from the Crab Nebula came from californium-249, an isotope with a half-life of 351 years — long enough that, if it had been produced in the 1054 explosion, it could still have been emitting gamma rays at the time of their study. In a paper co-authored with Haymes and Craddock, Clayton proposed that specific gamma-ray lines could be used to trace nucleosynthesis in supernovae.
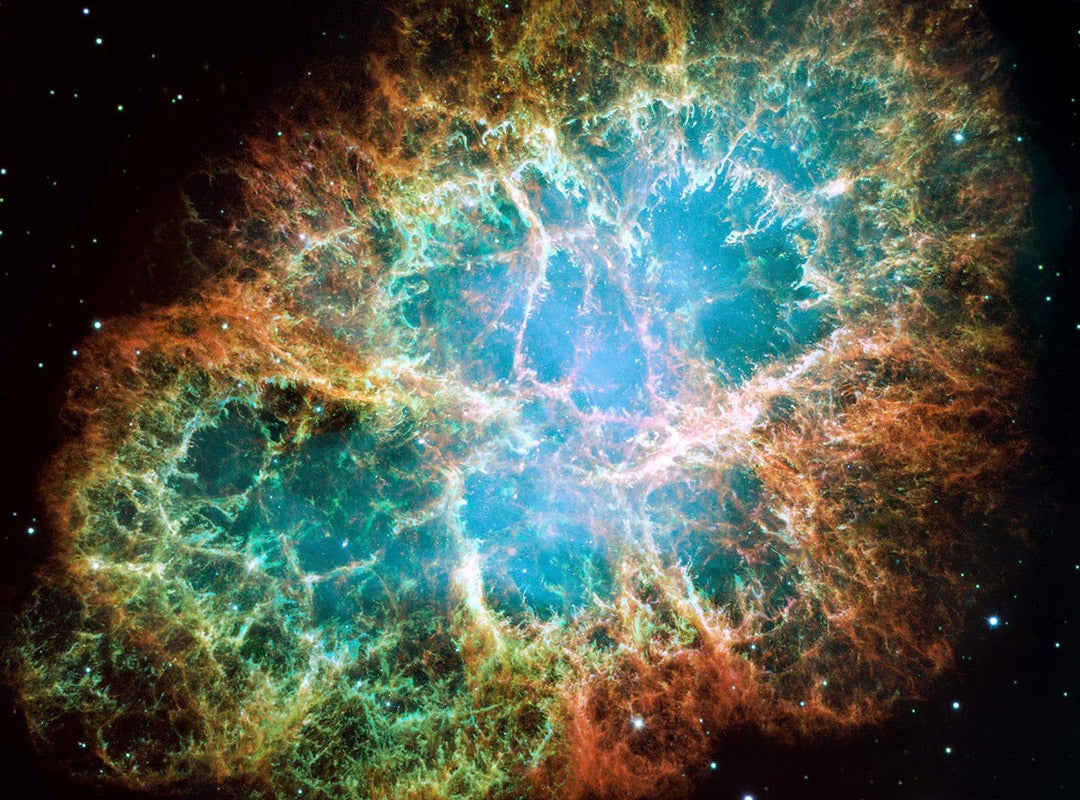
But Clayton had reservations. “Californium glowed and had the right half-life,” Woosley said. “But if that was what explained supernova light curves, we would be awash in uranium. There’d be a thousand times more uranium than there is.” Clayton included his reservations as a footnote to the paper and later recalled privately thinking, “No. This is not the explanation for the light curves, and we will not find these gamma-ray lines from the Crab Nebula.”
Clayton’s 1966-67 sabbatical year at Caltech set the stage for a major breakthrough. Working with Fowler, he set out to clarify the mechanism by which hot silicon nuclei in a star’s core transform into iron — a key step in understanding the evolution of a pre-supernova star. Iron held special importance as it is the most abundant of the common metals in the universe and one of the easiest elements to detect in stellar spectra.
The challenge was that silicon-28 nuclei cannot simply fuse directly to iron-56. At extreme stellar temperatures, silicon nuclei begin to break apart before reaching fusion conditions, releasing photons and alpha particles. Clayton and Fowler proposed a bold new model: under these conditions, silicon and other intermediate-mass nuclei enter a delicate state of ‘quasiequilibrium,’ a statistical balance among many nuclear species. Eventually, this equilibrium state breaks, and the dominant product is nickel-56, a radioactive isotope that decays first into cobalt-56 and then into stable iron-56.
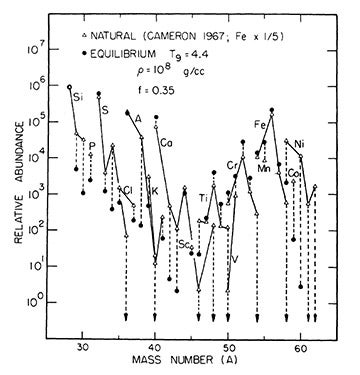
Their complete quasiequilibrium solution provided the first smooth, continuous description of the transformation from silicon-28 to iron-56, and successfully predicted the abundances of every isotope between atomic mass 28 and 62. Most significantly, it reframed the natural abundance of iron not as a direct product of stellar fusion, but as the legacy of a radioactive parent. As Clayton later recalled, “Our method worked, and the results were astonishing to the eye.”
In the spring semester of 1968, Clayton introduced his recent work on the transformation of silicon into radioactive nickel and its eventual decay into iron into the nucleosynthesis portion of his astrophysics course. After class one day, Gerald Fishman ’69, a graduate student working with Haymes to detect gamma-ray lines from radioactive nuclei with balloon-borne instruments, stayed behind to ask a question: Did any of the radioactive isotopes Clayton had mentioned emit gamma-ray lines? “My mind raced as Fishman and I walked back toward my office,” Clayton later wrote. “This might be the idea I had long awaited, the idea better than the californium hypothesis.”
Consulting a standard reference on nuclear decay, they found that nearly every decay of nickel-56 was accompanied by gamma ray emissions. At the blackboard, Clayton calculated that if a supernova ejected the expected mass of nickel-56, Haymes’ gamma-ray telescope should be able to detect the resulting gamma ray. "My feet practically left the floor," he recalled.
Clayton and Fishman, along with collaborator Stirling Colgate, worked quickly to draft a paper identifying which isotopes would produce detectable gamma-ray signatures and whether those gamma rays could escape the supernova to be detected from Earth. Their paper, “Gamma-Ray Lines from Young Supernova Remnants," would later be named one of the 50 most influential astronomy papers of the twentieth century by the American Astronomical Society. It marked the first prediction of a new kind of observational astronomy — one that would trace the radioactive fingerprints of stellar explosions using gamma-ray lines.

Woosley’s thesis research with Clayton was to develop simulations of supernova explosions, and these played a decisive role in confirming that iron-56 originates from the decay of radioactive nickel-56. His work showed that the observed abundances of iron-group elements could not be reproduced without including large amounts of nickel-56. Moreover, his calculated abundances of elements with atomic masses between 28 and 64 closely matched those found in the solar system. The rises and dips in the theoretical abundance curve were, in Clayton’s words, “stunning” in how closely they mirrored nature.
“Correct understanding of the origins of our chemical world can be discovered only once in the entire history of the human race,” Clayton later reflected. “What a thrill it was for Stan, for me and for [Rice colleague and collaborator] Dave Arnett to have been there, to have seen the stunning agreements that existed between our computer simulations of exploding supernova interiors and the jagged patterns of natural abundances.”
It would be decades before direct observational data confirmed these predictions.
“The challenge was that it was very difficult to build detectors that could see gamma rays, and the sensitivity just wasn’t great,” Woosley explained. ”We couldn’t see supernovae outside our own galaxy, and there hadn’t been one nearby in hundreds of years.” Still, Clayton continued to refine his models and push for more sensitive instruments. He argued that gamma-ray astronomy was the only way to observe “newly born nuclei” and that proof of ongoing nucleosynthesis in the universe would eventually come.

It did. On February 23, 1987, astronomers observed the explosion of a blue supergiant star in the Large Magellanic Cloud, 160,000 light-years from Earth. Supernova 1987A was the closest visible supernova since 1604, and it provided the perfect test for Clayton’s predictions. "I was breathless with excitement, having waited eighteen years for such an event," he later wrote.
Rice alumni played key roles in confirming the long-awaited signal. Mark Leising ’85 was part of a team at the Naval Research Laboratory that detected gamma-ray lines from Supernova 1987A using the Solar Maximum Mission’s sunward-pointing Gamma Ray Spectrometer. Fishman’s balloon-borne gamma-ray spectrometer, launched to the upper atmosphere in October 1987, was among several that also recorded gamma-ray energies from the explosion.
"This historic detection," Clayton wrote, "was the first occasion on which man had been able to confirm newly born nuclei in any exploding astronomical object." A new form of astronomy had been born.
The confirmation of Clayton’s decades-old prediction was a turning point in astrophysics, revealing the origins of the elements and opening a powerful new window into the universe through gamma-ray astronomy.
For Woosley and others who worked closely with Clayton, the memory of that time remains vivid. “We loved what we did. We loved this idea of making elements in the stars, and we loved bouncing ideas off of each other,” Woosley said. “He died last year, and it was a hard one. I thought he would live forever.”
Special thanks to Ishani Kaul ’25 for her contributions to the research and content for this article.
This work was made possible with support from the Fondren Fellows program.
